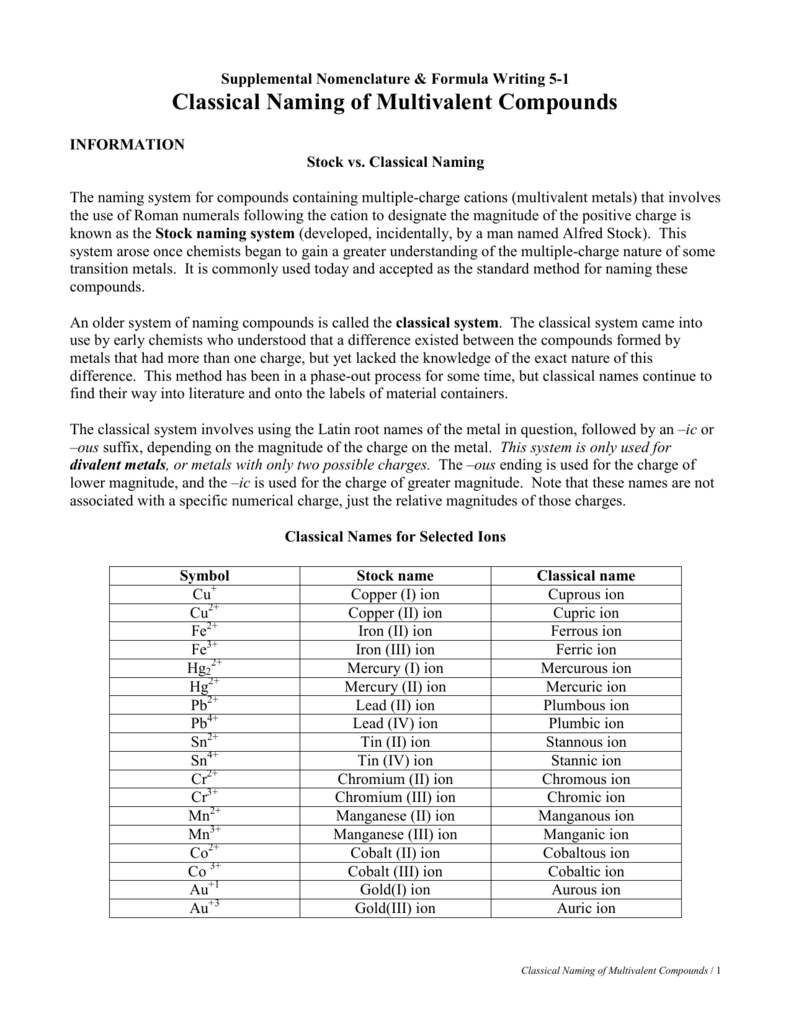
The Stock system allows the specification of transition metal ionic charge when naming ionic compounds. Roman numerals are used to indicate the amount of positive charge on the cation. The ratio of copper ions to oxide ions determines the name of a compound.
What is the stock system for naming compounds?
Stock nomenclature for inorganic compounds is a widely used system of chemical nomenclature developed by the German chemist Alfred Stock and first published in 1919. In the "Stock system", the oxidation states of some or all of the elements in a compound are indicated in parentheses by Roman numerals.
What is the naming system for ionic compounds?
For binary ionic compounds (ionic compounds that contain only two types of elements), the compounds are named by writing the name of the cation first followed by the name of the anion. For example, KCl, an ionic compound that contains K+ and Cl- ions, is named potassium chloride.
How do you do the stock system?
4:526:57The Stock System - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe Roman numeral here is telling you that the charge of the copper ion. Remember the Roman numeralMoreThe Roman numeral here is telling you that the charge of the copper ion. Remember the Roman numeral is the charge is +2. And chlorides charge is minus 1. So we have to get the charges to equal L.
Why do we need two naming systems for ionic compounds?
Iron, for example, can form two cations, each of which, when combined with the same anion, makes a different compound with unique physical and chemical properties. Thus, we need a different name for each iron ion to distinguish Fe 2+ from Fe 3+. The same issue arises for other ions with more than one possible charge.
How do you use roman numerals when naming ionic compounds?
In naming the transition metal ion, add a Roman numeral in parenthesis after the name of the transition metal ion. The Roman numeral must have the same value as the charge of the ion. In our example, the transition metal ion Fe2+ would have the name iron(II). Add the name of the anion to the transition metal ion.
What is stock method in chemistry?
The Stock Method of Naming An ionic compound is named first by its cation and then by its anion. The cation has the same name as its element. For example, K+1 is called the potassium ion, just as K is called the potassium atom.
What is stock notation example?
Stock notation is the representation of oxidation number by putting a Roman numeral in parenthesis after the symbol of the metal in molecular formula. So, correct notation is NO2: Nitrogen (IV) oxide. FeCl3 is wrong because it should have been Iron (III) chloride and not Iron (II) chloride.
When naming ionic compounds which of the following cations would be exceptions to the stock system?
There are several exceptions to the Stock system rule of using Roman numerals in the name of a cation. Two of the most common exceptions are zinc and silver.
When was Stock's system adopted?
In his own words, he considered the system to be "simple, clear, immediately intelligible, capable of the most general application.". In 1924, a German commission recommended Stock's system be adopted with some changes.
When did stock approve Roman numerals?
In 1934, Stock approved of the Roman numerals, but felt it better to keep the hyphen and drop the parenthesis. This suggestion has not been followed, but the Stock system remains in use world-wide. How do we name compounds when the cation of variable charge is involved?