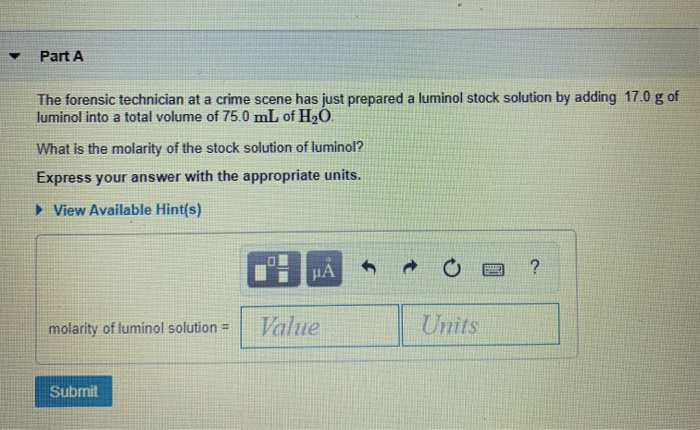
What is the molecular weight of the stock solution of luminol?
Aug 25, 2021 · 3 Answers molarity (M) = amount (mol) of solute / volume (L) of solution Via www.chemfinder.com, Luminol’s molecular formula is C8H7N3O2 and you can add up each element’s molar mass (use periodic table) to get to Luminol’s molecular weight or just use what chemfinder.com lists as the molecular weight.
How much luminol do you dilute before applying?
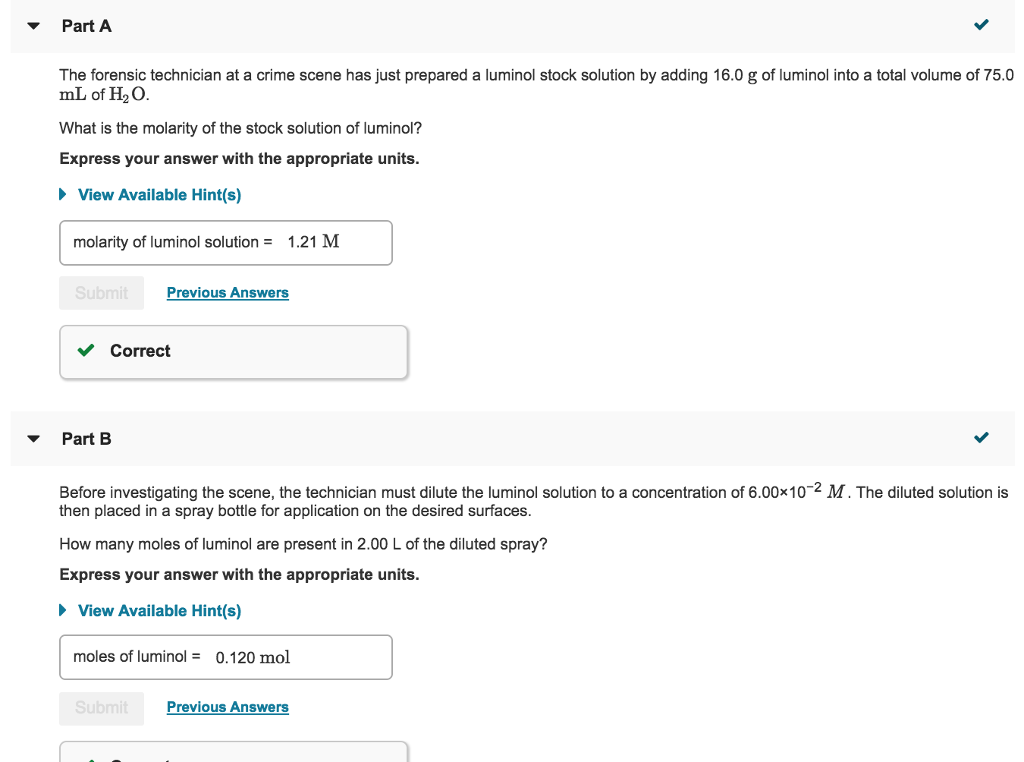
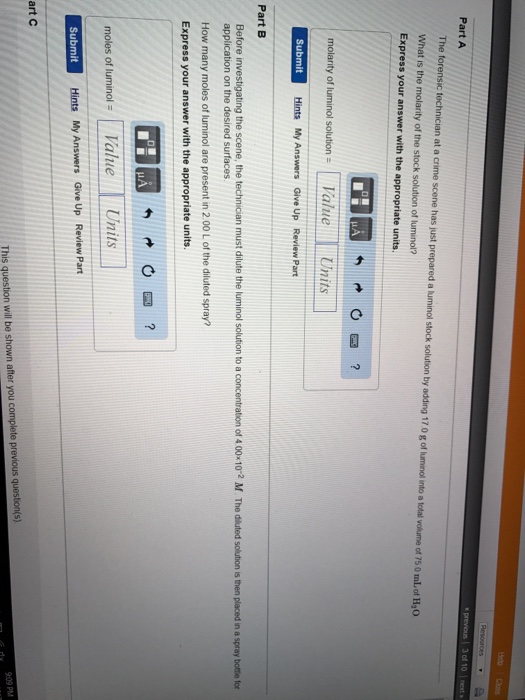
What is the molarity of the stock solution of luminol? Express your answer with the appropriate units. View Available Hint(s) molarity of luminol solution = 0.753 M Suomi Previous Answers Correct Part B Before investigating the scene, the technician must dilute the luminol solution to a concentration of 6.00x10-2 M.
How many moles of HCl are consumed by the stoichiometric equation?
question 5. part a) The forensic technician at a crime scene has just prepared a luminol stock solution by adding 15.0 g of luminol into a total volume of 75.0 mL of H2O. What is the molarity of the stock solution of luminol? Express your answer with the appropriate units. part b) Before investigating the scene, the technician must dilute the luminol
How to prepare a standard solution for C20H29FO3?
A standard solution is prepared for the analysis of fluoxymesterone (C20H29FO3), an anabolic steroid. A stock solution is first prepared by dissolving 10.0 mg of fluoxymestrone in enough water to give a total volume of 500.0 ml. A
What is the chemical used to detect blood?
The chemical 5-amino-2,3-dihydro-1,4-phthalazinedione, better known as luminol , is used by forensic scientists in analyzing crime scenes for the presence of washed-away blood. Luminol is so sensitive that it can detect blood that