What is the formula for calculating concentration?
2:004:24Concentration Formula & Calculations | Chemistry | Fuse School - YouTubeYouTubeStart of suggested clipEnd of suggested clipTogether if you want to find a concentration. You cover this and divide the number of moles by theMoreTogether if you want to find a concentration. You cover this and divide the number of moles by the volume. As try this now if you have two moles of salt and dissolve it in two liters of water use the
How do you calculate the concentration of a stock solution in mg mL?
Divide the mass in milligrams by volume in milliliters to find concentration in mg/mL. For example, if you have 8,000 milligrams of sugar dissolved in 200 milliliters of water, work out 8,000 ÷ 200 = 40. The concentration of the solution is 40 mg/mL.May 2, 2018
How do you calculate the concentration of a dilution?
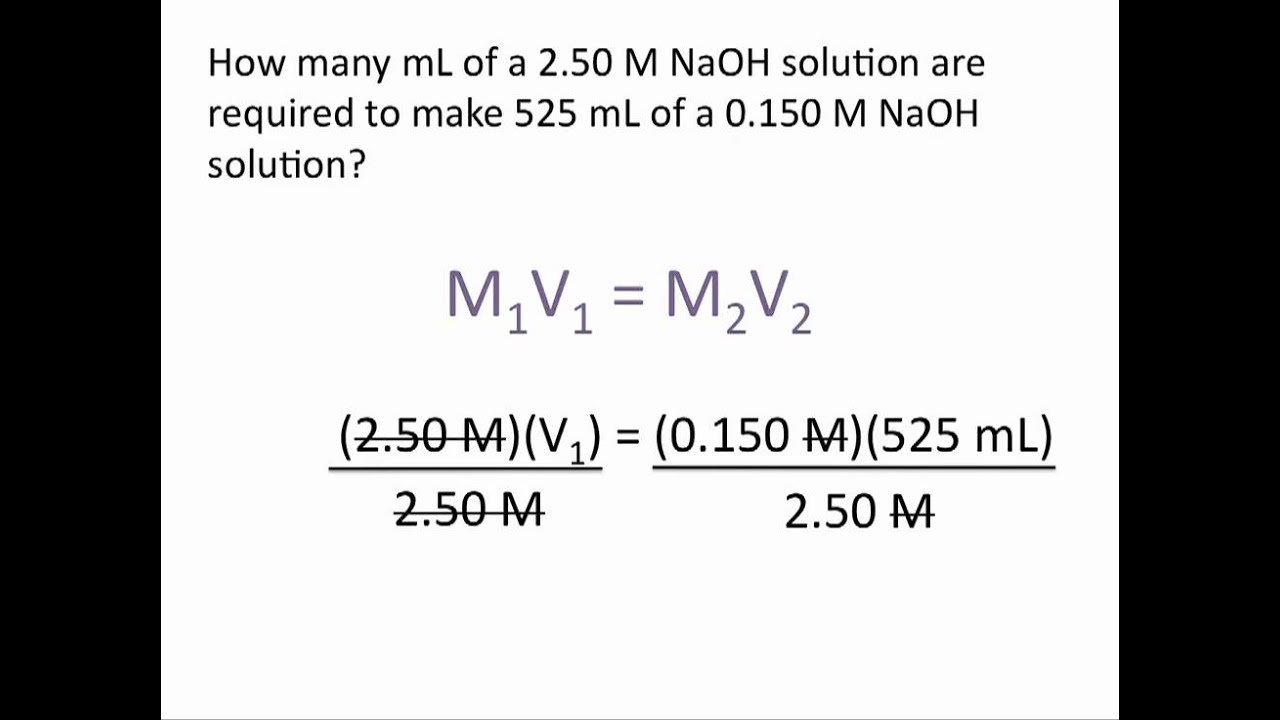
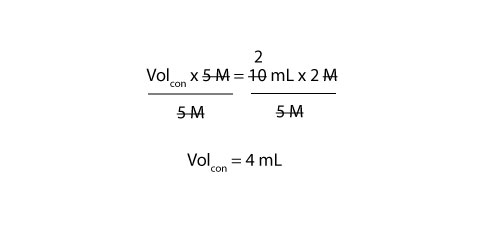
Most commonly, a solution 's concentration is expressed in terms of mass percent, mole fraction, molarity, molality, and normality. When calculating dilution factors, it is important that the units of volume and concentration remain consistent. Dilution calculations can be performed using the formula M1V1 = M2V2.
What is 50mg in ml?
Milligram to Milliliter Conversion TableWeight in Milligrams:Volume in Milliliters of:WaterGranulated Sugar50 mg0.05 ml0.071429 ml60 mg0.06 ml0.085714 ml70 mg0.07 ml0.1 ml37 more rows
How do you find the concentration of Mg 100 ml?
Concentration as %1% = 1 g in 100 ml ( =1000mg in 100ml = 10mg in 1 ml)50% = 50 g in 100 ml (= 500 mg in 1 ml = 5 g in 10 ml)
What is the concentration of a solution?
The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. A concentrated solution is one that has a relatively large amount of dissolved solute. A dilute solution is one that has a relatively small amount of dissolved solute.Jun 9, 2019
How do you find the concentration of a solution with absorbance?
In order to derive the concentration of a sample from its absorbance, additional information is required....Absorbance Measurements – the Quick Way to Determine Sample ConcentrationTransmission or transmittance (T) = I/I0 ... Absorbance (A) = log (I0/I) ... Absorbance (A) = C x L x Ɛ => Concentration (C) = A/(L x Ɛ)Sep 4, 2019
How do you calculate concentration from absorbance and dilution factor?
take the absorbance of sample (X) minus blank absorbance (Y) then multiply with the dilution factor (DF) and to get the concentration using the calibration curve.Jan 11, 2015
What are the units of concentration?
The most common units are molarity, molality, normality, mass percent, volume percent, and mole fraction. Here are step-by-step directions for calculating concentration, with examples.
What is concentration in science?
Concentration is an expression of how much solute is dissolved in a solvent in a chemical solution. There are multiple units of concentration. Which unit you use depends on how you intend to use the chemical solution.
How to find mass percent?
Calculate Mass Percent: mass solute divided by mass final solution multiplied by 100%.
What is volume percent?
Volume percent is the volume of solute per volume of solution. This unit is used when mixing together volumes of two solutions to prepare a new solution. When you mix solutions, the volumes aren't always additive, so volume percent is a good way to express concentration.
What is a mole fraction?
Mole fraction or molar fraction is the number of moles of one component of a solution divided by the total number of moles of all chemical species. The sum of all mole fractions adds up to 1. Note that moles cancel out when calculating mole fraction, so it is a unitless value.
What is stock solution?
posted on February 10, 2019. A stock or standard solution is a solution in which you accurately know its concentration. You can make stock solutions in the chemistry laboratory or buy from chemical manufacturers. Once you have a stock solution, you can prepare solutions of lower concentration by diluting the concentrated stock solution.
What does it mean to dilute a solution?
To dilute means to add a certain amount of solvent (water) to a certain amount of concentrated stock solution. If you add a certain amount of solvent to a certain amount of concentrated stock solution, you will notice that the amount of solute present in the stock solution is the same amount present in the dilute solution.
Is sulfuric acid more dense than water?
As you may know, sulfuric acid is much denser than water, so as you add it to water, its molecules are able to travel within and mix well with the water molecules. However, because water is less dense than sulfuric acid, if you add it to sulfuric acid, its molecules will create a barrier in which you have water on top and sulfuric acid below.
Calculate the dilution required to prepare a stock solution
The Tocris dilution calculator is a useful tool which allows you to calculate how to dilute a stock solution of known concentration. Enter C 1, C 2 & V 2 to calculate V 1.
New Technologies and Product Ranges at Tocris
We add approximately 250 new products a year, many of which are exclusive to Tocris. We have a dedicated team of PhD qualified product managers who assess all the latest technologies, bringing the most relevant to market first. View the products in our newest ranges below.
Supporting Scientists Since 1982
Tocris Bioscience has been supporting scientists for nearly 40 years! We supply and manufacture over 4500 gold standard and cutting-edge tools, used in every research field including respiratory system , cancer, immunology, cardiovascular, endocrinology, pain and inflammation, cell and gene therapy and stem cells.
How to find the concentration of a solution?
To calculate the concentration of a solution, start by converting the solute, or the substance being dissolved, into grams. If you're converting from milliliters, you may need to look up the solute's density and then multiply that by the volume to convert to grams. Next, convert the solvent to liters.
How to find the molar mass of a solute?
Add the atomic masses of the solute together to find the molar mass. Look at the elements in the chemical formula for the solute you’re using. List the atomic mass for each element in the solute since atomic and molar mass are the same. Add together the atomic masses from your solute to find the total molar mass.
What is the solute in chemistry?
The solute is the substance that you’re mixing in to form your solution. If you’re given the mass of the solute in your problem, write it down and be sure to label it with the correct units. If you need to find the mass of the solute, then weigh it on a lab scale and record the measurement.
What is stock solution?
A stock solution is a concentrated solution that will be diluted to some lower concentration, which is often referred to as a working or final concentration, for actual use. Stock solutions are used to save preparation time, reduce storage space, and improve the accuracy with which working lower concentration solutions are prepared.
What is the unit of concentration?
Molar concentration (also called molarity) is the number of molecules of a substance, expressed in mole units (see above), present in a certain volume of a solution. The most commonly used unit for molarity is the number of moles per liter, having the unit symbol mol/L or mol⋅dm −3. The value of molarity is also often expressed as "x-molar", e.g. 2.5 M (read as "2.5-molar") where "M" stands for mol/L.
What is the unit of measurement for amount of substance?
The mole is the unit of measurement for amount of substance. It is defined as exactly 6.02214076×10 23 (the Avogadro constant) particles, which may be atoms, molecules, ions, or electrons. The unit symbol is "mol".
What is a solution in chemistry?
A solution, in chemistry, is a homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent.
What is the measure of the number of moles of a solute present in 1 kg of solution?
Molality is a measure of number of moles of solute present in 1 kg of solvent. This contrasts with the definition of molarity which is based on a specified volume of the solution.
What is absorbance in physics?
Absorbance is a number that measures the attenuation of the transmitted light power in a solution. It is defined as the common logarithm of the ratio of incident to transmitted light power through a solution.
What is the molar extinction coefficient?
The molar extinction coefficient or molar attenuation coefficient is a measurement of how strongly a chemical species attenuates light at a given wavelength. The SI unit of molar attenuation coefficient is the square metre per mole (m 2 /mol), but in practice, quantities are usually expressed in terms of M −1 cm −1 or Lmol −1 cm −1.